Table Of Content
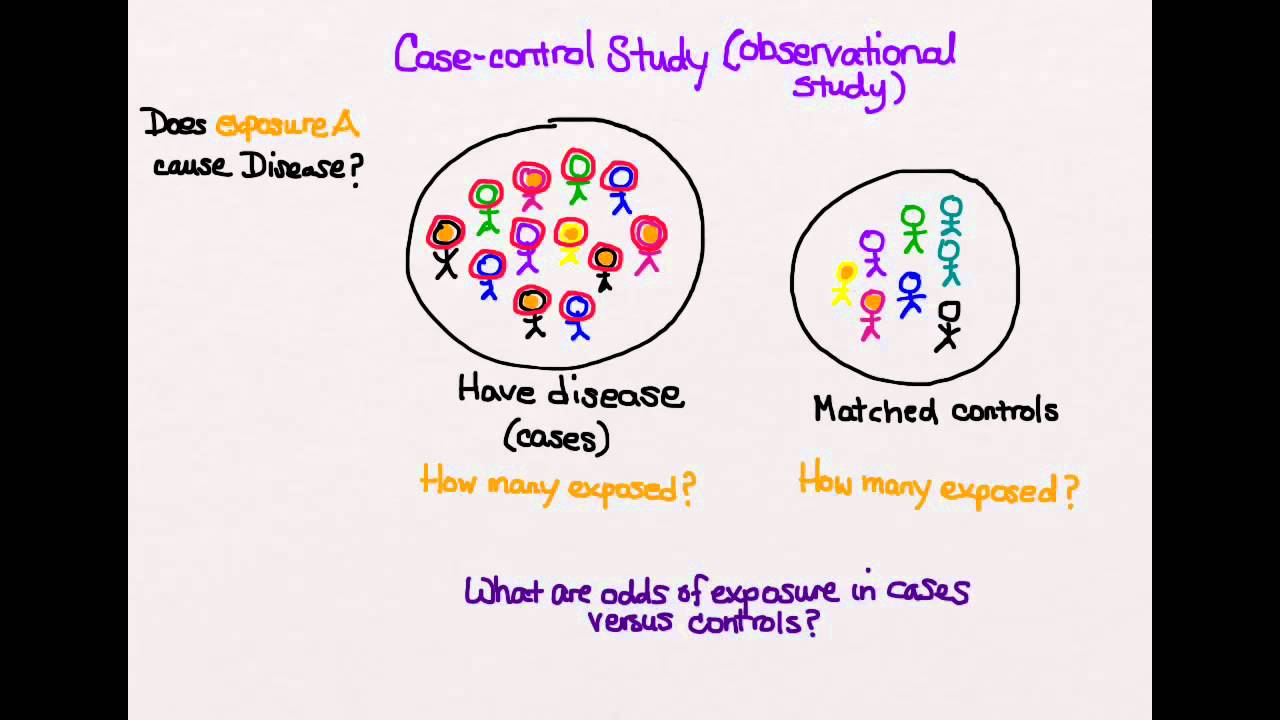
Case-control studies are a solid research method choice, but they come with distinct advantages and disadvantages. You would then collect information on any history of early life stress (e.g., abuse, neglect, trauma) for both the cases and controls and compare the two groups to determine if there is a relationship between early life stress and the risk of developing PTSD. You would then collect information on dietary intake of vitamin D for both the cases and controls and compare the two groups to determine if there is a relationship between vitamin D intake and the risk of developing osteoporosis. It’s important to remember that the case group is chosen because they already possess the attribute of interest.
Measuring Occurrence of Outcomes
In ‘The Dictionary of Epidemiology’ by Porta (2014), the authors have suggested that even though the term ‘retrospective’ was used for case-control studies, the study participants are often recruited prospectively. In fact, the study on risk factors for erysipelas (Pitché et al., 2015) was a prospective case case-control study. Thus, it is important to remember that the nature of the study (case-control or cohort) depends on the sampling method.
Survivor sampling
The researcher might find that those with Kaposi's sarcoma are more likely to have HIV, and thus conclude that HIV may be a risk factor for the development of Kaposi's sarcoma. Case-control studies in India tend to be poor in quality because they are based on smallsample sizes. Small samples do not have sufficient statistical power to adjust for themultitude of confounding variables that bedevil research in psychiatry. Large samples cannotbe identified because India does not as yet have large electronic health care databases as asource of data. Case-control studies are prevalent in all fields of medicine from nursing and pharmacy to use in public health and surgical patients. Case-control studies are important for each member of the health care team to not only understand their common occurrence in research but because each part of the health care team has parts to contribute to such studies.
Selecting the Study Cohort
The investigator must put a great deal of effort into creating a proper control group to bolster the strength of the case-control study as well as enhance their ability to find true and valid potential correlations between exposures and disease states. The primary challenge in designing a case-control study is the appropriate selection of cases and controls. Poor selection can result in confounding, in which correlations that are unrelated to the exposure exist between case and control subjects. Confounding in turn affects estimates of the association between disease and exposure, causing selection bias, which distorts OR figures. To overcome selection bias, controls typically are selected from the same source population as that used for the selection of cases.
For statistical reasons, however, there is little gained by including more than two controls per case. If we use controls from the past (time period when cases did not occur), then the controls are sometimes referred to historic controls. Sometimes, definition of a disease may be based on multiple criteria; thus, all these points should be explicitly stated in case definition. We strongly encourage the readers to read the papers to understand some practical aspects of case-control studies.
International diet quality index and revised diet quality index relationship with non-alcoholic fatty liver disease: a case ... - BMC Gastroenterology
International diet quality index and revised diet quality index relationship with non-alcoholic fatty liver disease: a case ....
Posted: Thu, 14 Dec 2023 08:00:00 GMT [source]
Case Control Study
Longitudinal change of gut microbiota in hypertensive disorders in pregnancy: a nested case–control and Mendelian ... - Nature.com
Longitudinal change of gut microbiota in hypertensive disorders in pregnancy: a nested case–control and Mendelian ....
Posted: Mon, 09 Oct 2023 07:00:00 GMT [source]
The potential for bias introduced by unmeasurable differences in patient selection, assessment, treatment, and follow-up is greater than in a rigorous prospective design. The immense power of prospective randomization to balance unknown and unmeasurable factors influencing outcome is not available to the retrospective researcher. Nonetheless, when a retrospective design is chosen for reasons of feasibility or practicality, it provides some of the strongest controls available under those constraints.
Quantifying the Drug–Outcome Association
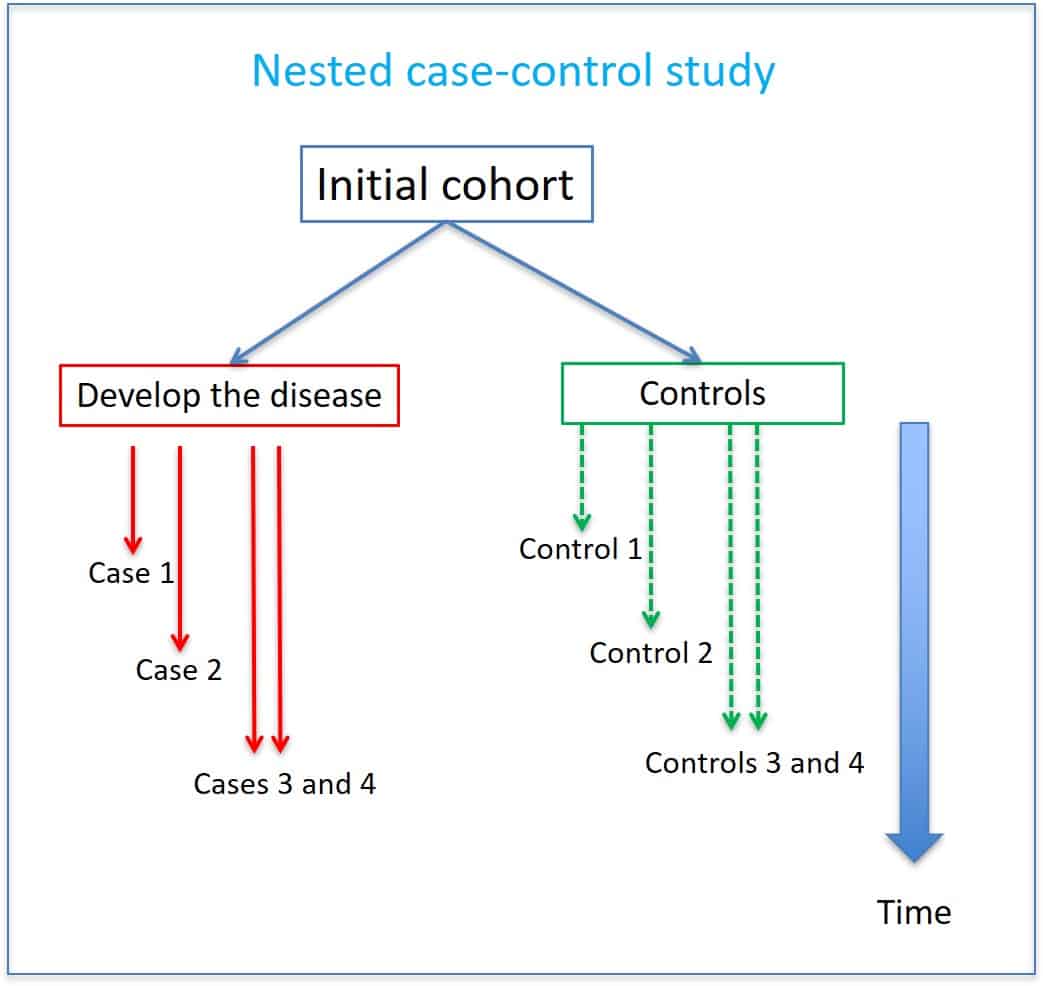
Like the cohort studies within which they are (at least conceptually) nested, case-control studies require an explicit definition of time zero, the time at which a choice is to be made between treatment strategies or protocols of interest [3]. Given a fixed cohort, time zero is generally determined by the defining event of the cohort (e.g., first diagnosis of a particular disease or having survived one year since diagnosis). However, while a fixed cohort may be ‘open’ to new members relative to calendar time, it is always ‘closed’ along the time axis on which all subject-specific time zeros are aligned. Cohort and case-control study designs are not “opposites” as are prospective vs.retrospective, or cross-sectional vs. longitudinal, or controlled vs. uncontrolled researchdesigns. Rather, like the randomized controlled and quasi-controlled designs, these designsare special kinds of research design in the controlled vs. uncontrolled classification. Notethat whereas a case-control study is always a special kind of controlled study, a cohortstudy can be classified under controlled or uncontrolled, depending on whether or not thereis a comparison group for the group of interest.
With these buttons at your fingertips, you can navigate your project timeline, set stills, and execute other commands with ease, significantly speeding up your workflow. Notably, some of these controls were previously exclusive to the larger DaVinci Resolve Mini and Advanced panels. Just in time for Apple’s May 7th event, Blackmagic announced the Micro Control Panel, a tiny, keyboard-sized controller that takes your iPad color grading to a whole new level.
As pharmacotherapy experts, pharmacists are continually updating their knowledge about drug effects. In addition to being knowledge users of research findings, pharmacists increasingly play a larger role in observational studies of drug effects. Observational studies are inherently nonexperimental and, unlike randomized clinical trials (RCTs), do not involve any manipulation (such as randomization) of the treatment and control groups by the investigator. The term ‘cohort’ may refer to either a ‘dynamic population’, or a ‘fixed cohort’, whose “membership is defined in a permanent fashion” and “determined by a single defining event and so becomes permanent” [9]. While it may sometimes be of interest to ask what would have happened with a dynamic cohort (e.g., the residents of a country) had it been subjected to one treatment protocol versus another, the results in this paper relate to fixed cohorts. Case-control studies are retrospective as researchers begin with an outcome and trace backward to investigate exposure; however, they differ from retrospective cohort studies.
The study involved comparing a group of former lifeguards that had developed cancer on their cheeks and noses (cases) to a group of lifeguards without this type of cancer (controls) and assess their prior exposure to zinc oxide or absorbent sunscreen lotions. Matching is often used in case-control control studies to ensure that the cases and controls are similar in certain characteristics. For example, in the smoking and lung cancer study, the authors selected controls that were similar in age and sex to carcinoma cases. These controls can be easily conducted the list of all individuals is available. For example, list from state identity cards, voter's registration list, etc., In the Tanning and melanoma study, the researchers used population controls. These controls are easy to recruit and they are also more likely to be similar to the cases in socio-economic status and other demographic factors.
An exciting feature for creators on the go is the integrated battery and Bluetooth connectivity. This makes the Micro Color Panel ideal for location shoots or editing suites with limited space. Even this small, it comes equipped with Bluetooth and a step tracker so that you can easily take control of your daily exercise.Effortlessly stylish, the GDB500 holds the key to all-new possibilities with G-SHOCK. Julia Simkus is a graduate of Princeton University with a Bachelor of Arts in Psychology. She is currently studying for a Master's Degree in Counseling for Mental Health and Wellness in September 2023. Consider a situation in which a large number of cases of post-operative endophthalmitis have occurred in a few weeks.
The factors (e.g., age, sex, time of hospitalisation) chosen to define how controls are to be similar to the cases are the ‘matching criteria’. The selected control group must be at similar risk of developing the outcome; it would not be appropriate to compare a group of controls who had traumatic corneal lacerations with cases who underwent elective intraocular surgery. In our example, controls could be defined as patients who underwent elective intraocular surgery during the same period of time.
It is important to remember that the concordant pairs (pairs in which the case and control are either both exposed or both not exposed) tell us nothing about the risk of exposure separately for cases or controls. We may have to use sampling methods (such as random digit dialing or multistage sampling methods) to recruit controls from the population. A main advantage is that these controls are likely to satisfy the ‘study-base’ principle (described above) as suggested by Wacholder and colleagues. Furthermore, many of these controls will not be inclined to participate in the study; thus, the response rate may be very low. On the other hand, cross-sectional studies collect data on a population at a single point in time. The goal here is to describe the characteristics of the population, such as their age, gender identity, or health status, and understand the distribution and relationships of these characteristics.
Because of their efficiency, they may also be ideal for preliminary investigation of a suspected risk factor for a common condition; conclusions may be used to justify a more costly and time-consuming longitudinal study later. The weaknesses of case–control studies include inefficiency for studying rare exposures, difficulty of selecting unbiased controls, and inability to directly calculate incidence rates of outcomes. One of the major strengths of a cohort study is that the temporal sequence—drug exposure preceding outcome—is explicit in the study design. The incidence of a particular outcome among persons exposed to a certain drug can be directly calculated using a cohort design. Cohort studies are also relatively efficient for studying rare exposures, and multiple outcomes may be assessed for a single exposure.
Formal statements and proofs are given in Supplementary Appendix C, which also includes a generalisation of the results of Table 2 to exact 1-to-M matching. While the focus in this section is on exact covariate matching, for partial matching we refer the reader to Supplementary Appendix D, where we consider parametric identification by way of conditional logistic regression. In this paper, we give an overview of how observational data obtained with case-control designs can be used to identify a number of causal estimands and, in doing so, recast historical case-control concepts, assumptions and principles in a modern and formal framework. Participants might be unable to remember when they were exposed or omit other details that are important for the study.
For example, Abdelmoneim and others29 specified a 120-day look-back period before the date of their cases (patients with acute coronary syndrome) to assess recent exposure to glyburide and gliclazide. Azoulay and others30 specified an exposure window of any time prior to a year before the date of cases in their study evaluating the association between pioglitazone and bladder cancer. If the investigators are collecting exposure data themselves, then outcome status should be blinded to study personnel. The case control study, while it has important strengths, is still a retrospective design with the problems that affect all retrospective studies. There may not be enough information available on enough subjects to analyze a particular factor of interest since the data collection was done after the fact.

No comments:
Post a Comment